April 30th 2018 - Biochemistry Questions and Answers â Enzyme Kinetics as an Approach to Understanding Mechanism Which of the following is true about Michaelis Menten kinetics a Newest kinetics Questions Biology Stack Exchange April 24th 2018 - Help Center Detailed answers to any questions you might have sides of the Michaelis Menten equation yields to study enzyme kinetics. An enzyme-machine analogy is described that has made the details of the Michaelis-Menten mechanism and the associated kinetics more accessible with minimal use of mathematics.
Michaelis Menten Kinetics And Briggs Haldane Kinetics
A particularly useful model for the kinetics of enzyme-catalyzed reactions was devised in 1913 by Leonor Michaelis and Maud Menten.
Michaelis menten approach to enzyme kinetics. Biochemical reactions involving a single substrate are often assumed to follow MichaelisMenten kinetics without regard to the models underlying assumptions. The Michaelis-Menten model 1 is the one of the simplest and best-known approaches to enzyme kinetics. An introduction to enzyme kinetics and the Michaelis-Menten plot the significance of Vmax and Km as well as the significance of pH and temperature on enzym.
We suggest that this inverted approach provides a general tool for kinetic analyses of interfacial enzyme reactions and that its analogy to established theory provides a bridge to the accumulated understanding of steady-state enzyme kinetics. The ES complex is formed by combining enzyme E with substrate S at rate constant k 1. MichaelisMenten kinetic parameters of immobilized enzymes can be determined using the same general approaches developed for the study of solubilized enzymes.
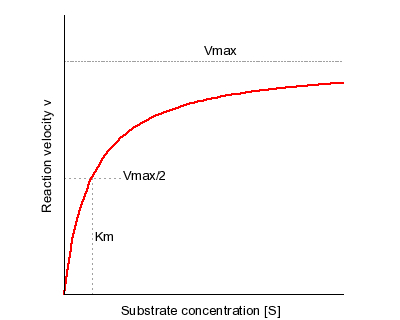
This includes the effect of temperature and pH on enzymatic activity. It is a pseudoequilibrium constant signifying the concentration of substrate at halfmaximal velocity of the enzyme. The Michaelis-Menten equation has been widely used for over a century to estimate the enzyme kinetic parameters from reaction progress curves of substrates which is.
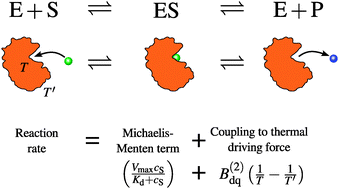
The MichaelisMenten Approachto Enzyme Kinetics. However Michaelis-Menten kinetics are inapplicable for most enzymes within a cell because most enzymes act on reactions that are close to equilibrium and they are unsuitable for most of the other enzymes within a cell because enzymes acting at far-from equilibrium reactions cannot afford to behave in such a lackadaisical fashion. The models are based on MichaelisMenten and first order kinetics which describe the reaction mechanism catalyzed by the enzymes.
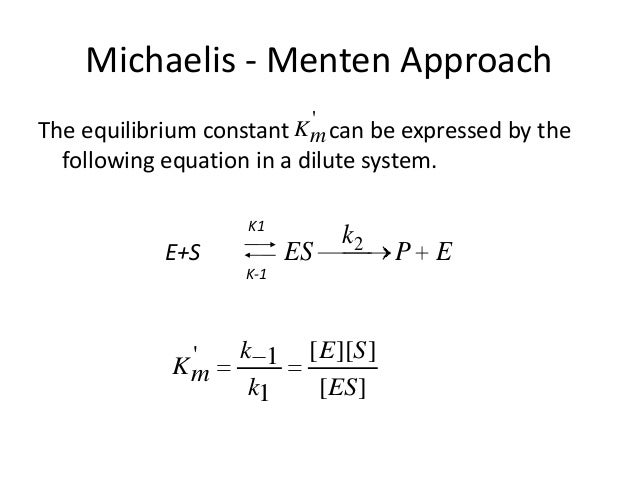
In biochemistry MichaelisMenten kinetics is one of the best-known models of enzyme kinetics. Approaches a limit where there is no dependence of rate on concentration see slide with limiting rate Vmax Leonor Michaelis and Maud Menten were among the first scientist to experiment with enzyme kinetics in a modern way controlling the pH of the solution etc. Condition that assumes that the rate at which the enzyme and the substrate associate to form enzymesubstrate complex k 1 ES is the same as the rate at which the latter dissociates back to its individual components k 1 ES.
What is MichaelisMenten kinetics model. The convention used for this slides is to use UPPERCASEfor the. Under this condition an inverse MichaelisMenten equation where the roles of enzyme and substrate had been swapped proved to be readily applicable.
When this is a competitive inhibitor only the waiting time is modified demonstrating that this compound binds at the same site as substrate. This original approach to Michaelis-Menten kinetics also permits a simple visualization of the events taking place at the recognitionbinding site of an enzyme when inhibitor is present. The standard approach to MichaelisMenten kinetics Calculate the initial rate of the reaction measured at varying substrate concentration from the linear portion of the.
It is still the basic model for nonallosteric enzymes and is widely used even. Fit the Eqn 8 or the polynomial 9 to the plotted reaction rate against concentration of the substrate see step 2. It takes the form of an equation relating reaction velocity to substrate concentration for a system where a substrate S binds reversibly to an enzyme E to form an enzyme-substrate complex ES which then reacts irreversibly to generate a product P and to regenerate the free enzyme E.
The enzyme interacts with the substrate by binding to its active site to form the enzyme-substrate. Michaelis-Menten Kinetics Introduction. When the inhibitor is a non-competitive inhibitor the situation is more.

Comparison Of Rate Laws And Their Resulting First Derivatives A Download Scientific Diagram

Enzyme Kinetics Michaelis Menten Kinetics Two Approaches 1 Rapid Equilibrium Approach 2 Quasi Steady State Approach Assumptions Total Enzyme Concentration

Enzyme Kinetics Michaelis Menten Kinetics Ppt Video Online Download

Enzyme Kinetics Michaelis Menten Kinetics Ppt Video Online Download

Michaelis Menten Kinetics Wikiwand
Michaelis Menten Kinetics Under Non Isothermal Conditions Physical Chemistry Chemical Physics Rsc Publishing
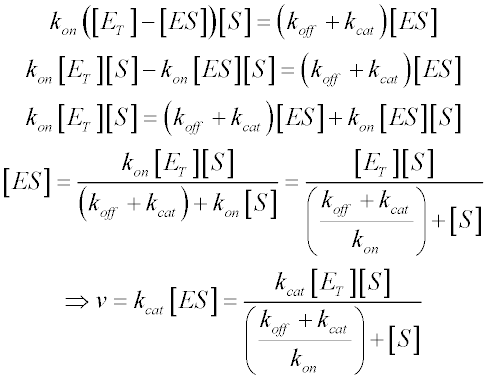
Michaelis Menten Kinetics And Briggs Haldane Kinetics

Atypical Michaelis Menten Kinetics In Cytochrome P450 Enzymes A Focus On Substrate Inhibition Sciencedirect
Michaelis Menten Plot Of The Dependence Of The Catalytic Current As A Download Scientific Diagram

Theory On The Rate Equation Of Michaelis Menten Type Single Substrate Enzyme Catalyzed Reactions Biorxiv

Michaelis Menten Kinetics Wikipedia
Michaelis Menten Kinetics Wikipedia

Estimating Kinetic Constants In The Michaelis Menten Model From One Enzymatic Assay Using Approximate Bayesian Computation Tomczak 2019 Febs Letters Wiley Online Library