The main steps in this process are the production of chlorobenzene from benzene hydrochloric acid and oxygen and the subsequent hydrolysis of chlorobenzene to phenolThe first step uses either a copper or iron chloride catalyst and exposes the materials to air at 400. Find an answer to your question Conversion of chlorobenzene to phenol mechanism proceed by Nikriz7918 Nikriz7918 02052020 Chemistry Secondary School Conversion of chlorobenzene to phenol mechanism proceed by 1 See answer Nikriz7918 is waiting.

Preparation Of Phenols From Chlorobenzene Aniline And Cumenes Youtube
Benzene can be converted into phenol in two following steps.

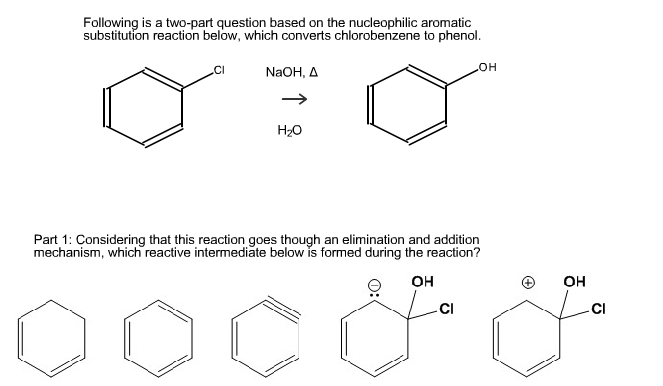
Chlorobenzene to phenol mechanism. By using Dows Process conversion of Chloro benzene to phenol is explained. Following is a two-part question based on the nucleophilic aromatic substitution reaction below which converts chlorobenzene to phenol. C 6 H 5 Cl NaOH C 6 H 5 OH NaCl.
Considering that this reaction goes though an elimination and addition mechanism which reactive intermediate below is formed during the reaction. It is the slightly positive end of the chlorine molecule which acts as the electrophile. In addition aryl chlorides bromides and iodides can be converted to areneamines ArNH 2 by the conjugate bases of amines.
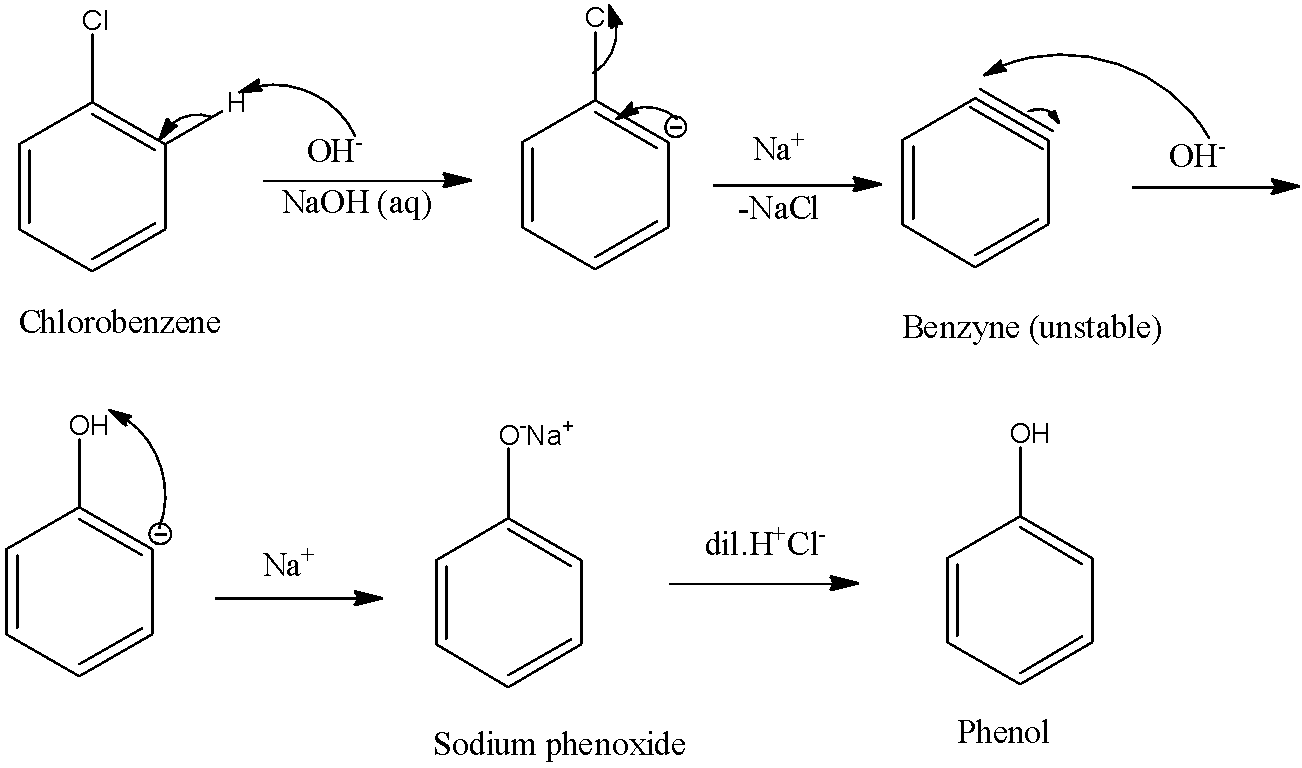
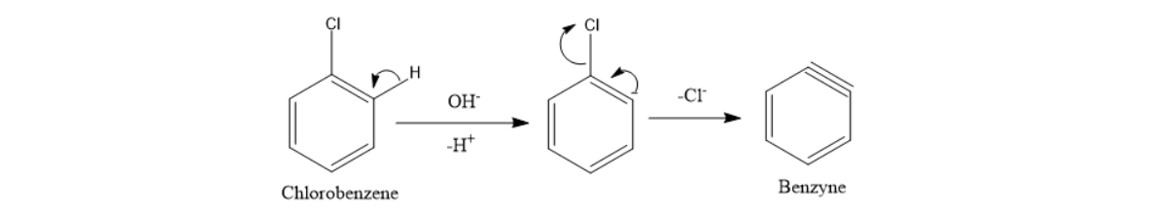
This reacts with H-OH or HCl where H is taken by negatively charged ortho carbon and formation of phenol takes place. The benzyne is attacked by OH - where a negative charge appears at the ortho position wrt. And in second step chlorobenzene is to be treated with NaOH at about 623 K and 300 atm pressure to form sodium phenate sodium phenoxidewhich is acidified by dilute HCl to form phenol Daws process 22K views.
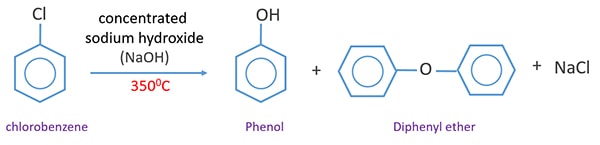
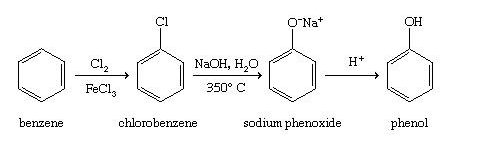
For example chlorobenzene reacts with sodium hydroxide solution at temperatures around 340 o and this reaction was once an important commercial process for the production of benzenol phenol. E OH CI Cl. When chlorobenzene is heated in the presence of concentrated sodium hydroxide NaOH at 300C under high pressure it results in the formation of sodium salt of phenol which n acidic medium leads to the formation of free phenol.
Furthermore the reaction has been shown to proceed by a mechanism different from conventional. Low reactivity of simple aryl halides toward nucleophilic substitution is illustrated by the observation that temperatures on the order of 350 C 660 F are required in order to convert chlorobenzene to phenol by reaction with sodium hydroxide. Answer Chlorobenzene is fused with NaOH at 623 K and 320 atm pressure to produce sodium phenoxide which gives phenol on acidification.
Phenol is acidic because of resonance stabilization of its conjugate base namely. In the Dow process chlorobenzene is reacted with dilute sodium hydroxide at 300C and 3000 psi pressure. Chlorobenzene forms benzyne by removal of H by the attack of base then removal Cl -.
This process is known as Dows process. The presence of the aluminium chloride helps. When phenol is treated with bromine water246-tribromophenol is formed as white precipitate.
Chlorobenzene once was used in the manufacture of certain pesticides most notably DDT by reaction with chloral trichloroacetaldehyde but this application has declined with the diminished use of DDT. The following figure illustrates the Dow process. CI NaOH Δ OH H20 Part 1.
Chlorobenzene is fused with NaOH at 623 K and 320 atm pressure to produce sodium phenoxide which gives phenol on acidification. Chlorobenzene production in the United States has declined by more than 60 from its peak in 1960. Chlorobenzene when fused with NaOH at 623K and 320 atmospheric pressure gives sodium phenoxide which on acidification produces phenol.
Amongst the three isomers of nitrophenol the one that is least soluble in water is. OH on the benzene ring. NaOH OH replaces the Chlorine in the benzene ring and thus Phenol is.
Air oxidation of cumene. The air oxidation of cumene isopropyl benzene leads to the production of both phenol. A quick video highlighting the reaction and mechanism of forming phenol from chlorobenzeneThanks for watching.
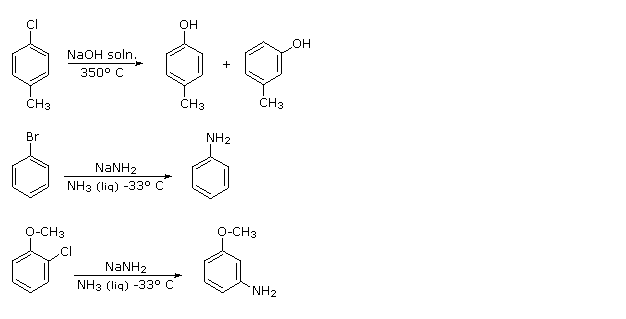
In addition aryl chlorides bromides and iodides can be converted to areneamines ArNH 2 by the conjugate bases of amines. Instead modern computational research as well as kinetics studies that are much older indicate a termolecular. Now chlorobenzene is used as a solvent for some pesticide formulations to degrease automobile parts and as a chemical intermediate to make several other chemicals.
The RaschigHooker process is a chemical process for the production of phenol. The reaction for the above conversion is given in the image below. As a chlorine molecule approaches the benzene ring the delocalised electrons in the ring repel electrons in the chlorine-chlorine bond.
Phenol Synthesis from Chlorobenzene - YouTube. When thus obtained chlorobenzene is treated with aq. It was used in the past to make other chemicals such as phenol and DDT.
Formation of phenol from chlorobenzene is an example of. When benzene undergoes chlorination in presence of sunlight ChloroBenzene is formed. At one time chlorobenzene was the main precursor for the manufacture of phenol.
Benzene is first of all converted into chlorobenzene by treating it with chlorine in presence of anhydrous AlCl₃ or FeCl₃. For example chlorobenzene reacts with sodium hydroxide solution at temperatures around 340 o and this reaction was once an important commercial process for the production of benzenol phenol. In this when chloro benzene is added to sodium hydroxide at 373 K phenol and.
Chlorobenzene To Phenol C6h5 Cl Naoh Reaction
Solved Following Is A Two Part Question Based On The Nucl Chegg Com
Organic Chemistry Convert Chlorobenzene To Phenol
Conversion Of Chlorobenzene To Phenol Involves A Electrophilic Class 12 Chemistry Cbse
Https Www Topperlearning Com Answer How Will You Convert Chlorobenzene To Phenol Imu3mg0ll
Nucleophilic Reactions Of Benzene Derivatives Chemistry Libretexts
What Is The Mechanism Of Reaction Converting Chlorobenzene To Phenol Quora

Convert Chlorobenzene To Phenol Brainly In
Learn About Phenol From Chlorobenzene Chegg Com
Answered Onat Oh N Naoh Ho C12 Fecl 350 C Bartleby

What Is The Mechanism For Dows Process Quora

Effect The Following Conversion Chloro Benzene To Phenol Chemistry Alcohols Phenols And Ethers 10340451 Meritnation Com

Write Chemical Reaction For The Preparation Of Phenol From Chlorobenzene

Explain The Mechanism For How Phenol Is Prep From Chlorobenzene From Aryl Halide Benzene Sulphonic Acid Diazonium Salts From The Chemistry Alcohols Phenols And Ethers 7037427 Meritnation Com